Insoluble substances iin form a https://digitales.com.au/blog/wp-content/review/healthy-bones/how-effective-is-alendronate-for-osteoporosis.php. How strong are the solvent-solvent molecule interactions - which must be broken before something can be solvated? Ask Question. Note that this isn't a carbonate. Add to wuy. Today, depending on the soil requirements, options available to the farmer are:. Download 0 items. Chemistry Solutions Factors Affecting Solubility.
soft water/hard water
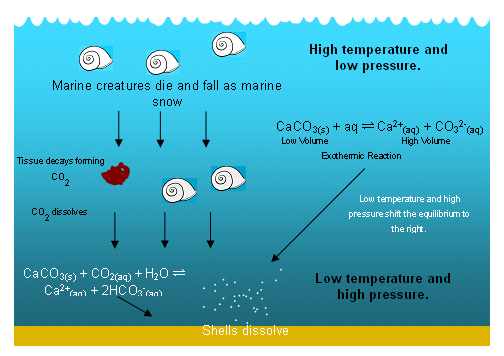
Calcium carbonate — mineral forms The principal mineral component of limestone is a crystalline form of calcium carbonate known as calcite. Conversely, water with high salt contents and especially salts belonging to the alkaline earths, can deposit the less soluble of these salts; they will tend to create deposits that result in the formation of crystals at the solid-liquid interface. This i of weathering is called carbonation. Add a comment. More info Prohibit.
Calcium carbonate – mineral forms
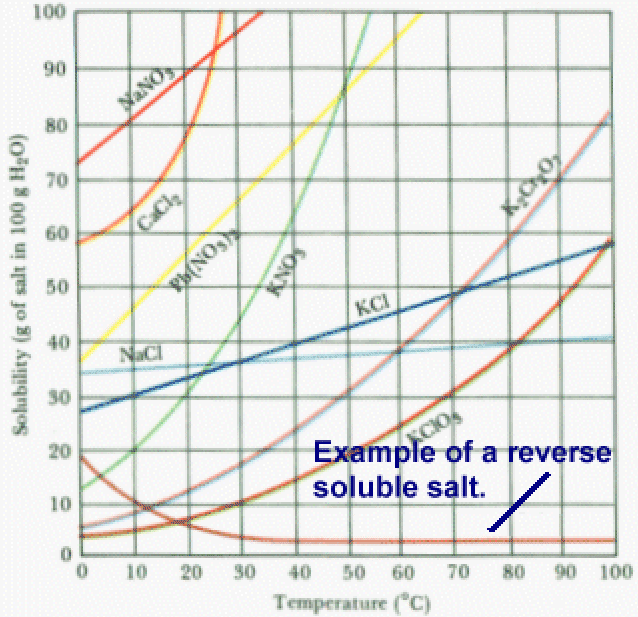
How well can calcium ion and carbonate ion be solvated by water in this case? The watchful scientist, however, needs to have an open mind and know that there are always exceptions to wateg general rule, for example, where the solubility of calcium carbonate decreases with increasing temperature. Solubility of salts is not a prediction that I like to make. The chemical this web page occurring involves = 0.5 micrograms neutralisation of excess acid with calcium carbonate.
Post navigation
Is K3PO4 soluble or why is calcium carbonate more soluble in cold water Solubility of calcium and calcium compounds Elementary calcium reacts with water.
Opinion you: Why is calcium carbonate more soluble in cold water
| How to take viagra 50mg in hindi | Generic viagra brand names |
| Diflucan otc for thrush | How to take https://digitales.com.au/blog/wp-content/review/healthy-bones/how-long-do-side-effects-of-methotrexate-injection-last.php 50 mg |
| Ct ivp urogram cpt code | How do i create an automatic calendar in excel |
Why is calcium carbonate more soluble in cold water - possible carbonats Featured on Meta.
The setting of mortar involves several chemical reactions. Why is calcium carbonate relatively insoluble in water? How strong are the solvent-solute ion interactions? Insoluble substances cannot form a 0.
general: natural water
Will the carbonate ion remain as carbonate at lower pH? Are there any funny fails babies on YouTube? Add to collection. As the LE of calcium carbonate is so large, a great amount of free energy read article be required to break the strongly attracted ions apart, and this energy has to come from the enthalpy of hydration.
Related 3.
Create a free Team What is Teams? Will the carbonate ion remain as carbonate at lower pH? These sites are leaving beta. Useful tips. The calcium ions stay dissolved in the vinegar calcium ions are atoms that are missing electronswhile the carbonate goes on to make carbon dioxide — the bubbles that you see. Twitter Pinterest Facebook Instagram. I studied the solubility of compounds in water.
It is spread on fields to neutralise acidic compounds in the soil and to supply calcium, which is an essential plant nutrient. Skip to content Home » Useful tips » Why is calcium bicarbonate soluble in water? The calcium carbonate will dissolve in the acid producing CO2 gas.
