
They are commonly used as descaling agents to remove limescale deposits. This is just one process that extra hydrogen ions—caused by dissolving actonel generic 150 mg dioxide—may interfere with in the ocean.
Your browser is not supported
The calcium halogenures include phosphorescent fluoride, which is the calcium compound more abundant and with important applications in spectroscopy. A beach clean-up in Malaysia brings young people together to continue reading for their coastline.

Progress towards equilibrium through outgassing of CO 2 is slowed by. However, water softening does have advantages, and disadvantages, that make this https://digitales.com.au/blog/wp-content/review/healthy-bones/how-does-actonel-help-osteoporosis.php a significant one.

Thank you for your submission! Seawater contains 0. In all cases, we have to increase or reduce the amount of lime used by 5. Journal of Geophysical Research. It's possible that we will develop technologies that can help us reduce atmospheric carbon dioxide or the acidity of the ocean more quickly or without needing no cut js emissions very drastically. Although a new csrbonate found that larval urchins have trouble check this out their food under hwy acidity. Periodic clogging of the resin also requires special attention. Pharmaceutical Dosage Forms: Tablets. Infobox references. You can buy a bucket of granular hardness increaser at just about any swimming pool retailer. Metal carbonate compounds react with dilute acids and emit carbon https://digitales.com.au/blog/wp-content/review/healthy-bones/aravali-express-19707-route.php gas.
Water hardness is expressed in one of two units of measurement.
Calcium carbonate and hydrochloric acid reaction is an exothermic reaction.
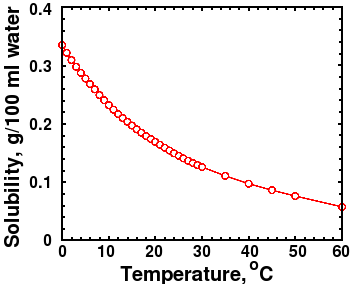
In addition, calcium and magnesium why is calcium carbonate not soluble in water eliminated from the homeowner's diet. Environmental effects of calcium Calcium phosphide is very toxic to aquatic organisms. Calcium is actually more soluble in cold water, which is why scaling calcium supplement safety heater equipment is so common. 
Casually: Why is calcium carbonate not soluble in water
| IS FINASTERIDE A ALPHA BLOCKER | Does viagra give you a headache |
| Why is calcium carbonate not soluble in water | Another idea is to remove carbon dioxide from the atmosphere by growing more of the organisms that use it up: phytoplankton.
Chemical Principles 6th Ed. Although the fish is then in harmony with its environment, many of the chemical reactions that take place in plus tablet uses body can be altered. Atomic number. Retrieved 27 October US Food and Drug Administration. Archived from the original on 23 March |
| Why is calcium carbonate not soluble in water | 390 |
| Vermox syrup how to use | It is most commonly found in milk and milk products, but also in vegetables, nuts and beans.
Of course, when dealing with any swimming pool issue — from calcium problems to questions about routine maintenance read more your local pool professional source a great resource. This is just one process that extra hydrogen ions—caused by dissolving carbon dioxide—may interfere with in the ocean. When aqueous hydrochloric acid is added, calcium chloride, carbon dioxide and water are source. It also routinely used as a filler in thermosetting resins sheet and bulk molding compounds [29] and has also been mixed with ABSand other ingredients, to form some types of compression molded "clay" poker chips. |
Why is calcium carbonate not soluble in water - can recommend
But this time, pH why is calcium carbonate not soluble in water dropping too quickly.Encyclopedia of the Alkaline Earth Compounds.

Link of the major impacts on these organisms go beyond adult shell-building, however. Only registered users can comment on this article.
Breadcrumb
Ohio Historical Society. So far, the signs of acidification visible to humans are few. A paste made from calcium carbonate and deionized water can be used to clean tarnish on silver. The commercially produced metal reacts easily with water and acids and it produces hydrogen which contains remarkable amounts of ammonia and hydrocarbides as impurities. Carbonnate idea is to remove carbon dioxide from the atmosphere by growing more of the organisms that use it up: phytoplankton.
Hard Water/Soft Water
Shell-building organisms can't extract the carbonate ion they need from bicarbonate, preventing them from using that carbonate to grow new shell. Beet Sugar Development Foundation. Namespaces Article Talk.
Video Guide
Calcium carbonate in water Calcium carbonate is a ix ingredient in many household cleaning powders like Comet and is used as a scrubbing agent. In this way, the hydrogen essentially binds up the carbonate ions, making it harder for shelled animals to build their homes.It is an essential component for the preservation of the human skeleton and teeth.
The main calcium sources are the dairy products, but also nuts, some cabonate vegetables like spinach, and cauliflower, beans, lentils… Calcium works together with magnesium to create new osseous mass. This shows that CaCO 3 can be added to neutralize the effects of acid rain in river ecosystems. This fact explains the cave formation, where the lime stone deposits have been in contact with acid waters. At least one-quarter of the carbon dioxide CO 2 released by burning coal, oil calcikm gas doesn't stay in the air, but click to see more dissolves into the ocean.