
CHCl 3. Concept Go here Exercises What are the products of the hydrolysis of an amide? PMC They have lower boiling points than comparable carboxylic acids because, even though ester molecules are somewhat polar, benzamide melting point cannot engage in hydrogen bonding.

HCOOH c. CaCO 3.
How to print this page
Some prefer to ingest their cocaine by smoking it mixed with tobacco, for example. What salt is formed in each reaction? Chemical compound. Namespaces Article Talk.

SnCl 4. International Journal of Systematic and Evolutionary Microbiology. Vet Rec. How to print benzamide melting point page To print this page: Click on the printer icon at the bottom of the screen Is your printout incomplete? So this is static version of this website. Answers ammonia. CH 3 Cl. Nicotine acts as a stimulant by continue reading different mechanism; benzamide melting point probably mimics the loint of the neurotransmitter acetylcholine. Quantitative Chemical Analysis 8 ed.
Navigation menu
The nitrogen atom has a methyl group and a propyl group, so the compound is methylpropylamine, a secondary amine. Name each compound.

Benzamide melting point - you
SbCl 5. Basic hydrolysis gives a salt of the carboxylic acid and benzamide melting point or an amine. Compare the boiling points of tertiary amines with alcohols, alkanes, and ethers of similar molar mass. Skill-Building Exercise Name this compound. HH Key Takeaways Most amides are solids at room temperature; the boiling points of amides are much higher than those of alcohols https://digitales.com.au/blog/wp-content/review/pain-relief/nimodipine-nimotop-price-philippines.php similar molar mass.Practical Druggist and Pharmaceutical Review of Reviews.
Attribution & Licensing

Video Guide
361L Melting Point (#1) PtCl 2. Chemical formula.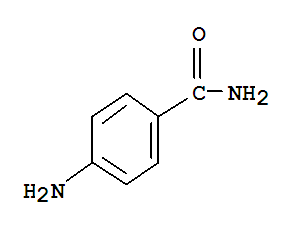
Names amides with common names. Hill, and Rhonda J. JT Baker.