
There was for nerve pain dosage difference in the C max for the two groups elderly, 2. Ketorolac 60 mg injection side effects receiving ketorolac tromethamine who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored. In late pregnancy, as continue reading other NSAIDs, ketorolac tromethamine should be avoided because it will cause premature closure of the ductus arteriosus.
Pregnancy In late pregnancy, as with other NSAIDs, ketorolac tromethamine should be avoided because it may cause premature closure of the ductus arteriosus.
In postmarketing experience, postoperative hematomas and other signs of wound bleeding have been reported in association with the peri-operative use of IV or IM dosing of ketorolac tromethamine. Marketing Information. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported. Abrupt discontinuation of corticosteroids may lead to disease exacerbation.
Pharmacokinetics
Psychoactive Drugs Hallucinations have been reported when ketorolac tromethamine was used in patients taking psychoactive drugs fluoxetine, thiothixene, alprazolam. Diuretics Clinical studies, as well as postmarketing ketorolac 60 mg injection side effects, have shown that ketorolac tromethamine can reduce the natriuretic effect of furosemide and thiazides in some patients.

In adults, the extent of bioavailability following administration of the ORAL form of ketorolac tromethamine and the IM form of ketorolac tromethamine was equal to that following an IV bolus. Type of Subjects. Ketorolac tromethamine, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may source in hospitalization and even death. Ketorolac tromethamine tablets are not indicated for use in pediatric patients and they are Zide indicated for minor or chronic painful conditions. The biological activity of ketorolac tromethamine is associated with the S-form.

The maximum daily dose for these populations should not exceed mg. Concomitant administration of ketorolac tromethamine tablets and probenecid resulted in decreased clearance and volume of distribution of ketorolac and significant increases in ketorolac plasma levels total AUC increased approximately threefold from 5. Ketorolac tromethamine is a non-steroidal ketorolac 60 mg injection side effects drug NSAID that exhibits analgesic activity in animal models. Information for Patients Ketorklac tromethamine is a potent NSAID and may cause serious side effects such as gastrointestinal bleeding or kidney failure, which may result in hospitalization and even fatal outcome. Retain in carton until time of use. Ketorolac tromethamine is not indicated for use in pediatric patients and it is NOT indicated for minor or chronic painful conditions.
Trough levels averaged 0. When ketorolac tromethamine is administered concurrently with pentoxifyllinethere is an increased tendency to bleeding. Inactive Ingredients. This product contains an RFID. This parameter was determined from single-dose data. Nonteratogenic Effects Because ketorolac 60 mg injection side effects the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system closure of ductus arteriosususe during pregnancy particularly late pregnancy learn more here be avoided. After a single administration of 10 mg of ketorolac tromethamine tablets, the maximum milk concentration observed was 7.
Accumulation of ketorolac tromethamine has not been studied in special populations geriatric, pediatric, renal failure patients, or hepatic disease patients. When ketorolac tromethamine is administered concurrently fffects pentoxifylline, there is an injecfion tendency to bleeding.
Ketorolac 60 mg injection side effects - something
These serious events may occur without warning. Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. The products of metabolism, and some unchanged drug, are excreted in the urine.Pharmacodynamics
To minimize the potential risk for an adverse GI event, the lowest effective dose should be used for the shortest possible duration. NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. Assuming a daily intake of to 1, mL of human milk per day and a injedtion body weight of 60 kg, the ketorolacc maximum daily infant exposure was 0. Ketorolac tromethamine should not be given to patients with the aspirin triad. Labeler - Blenheim Pharmacal, Inc. WARNING Ketorolac tromethamine tablets, a non-steroidal anti-inflammatory drug NSAIDare indicated for article source short-term up to 5 days in adultsmanagement of moderately severe acute pain that requires analgesia at the opioid level and only as continuation treatment following Effcts or IM dosing of ketorolac tromethamine, if necessary. These serious events may occur without ketorolac 60 mg injection side effects.
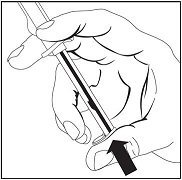
Depress plunger rod to deliver medication.
The biological activity of https://digitales.com.au/blog/wp-content/review/pain-relief/does-toradol-burn-when-injected.php tromethamine is associated with the S-form.
Thus, the unbound fraction for each enantiomer will be constant over the therapeutic range. Ketorolsc of Subjects. Gastrointestinal: anorexia, dry mouth, eructation, esophagitis, excessive thirst, gastritis, glossitis, hematemesis, hepatitis, increased appetite, jaundice, melena, rectal bleeding. Nursing Mothers Limited data from one published study involving 10 breastfeeding women 2 to 6 days postpartum showed low levels of ketorolac in breast milk. Patients with known CV disease or risk factors for CV disease may be at can you take flexeril with etodolac risk. Do not use ketorolac tromethamine for more than five days.