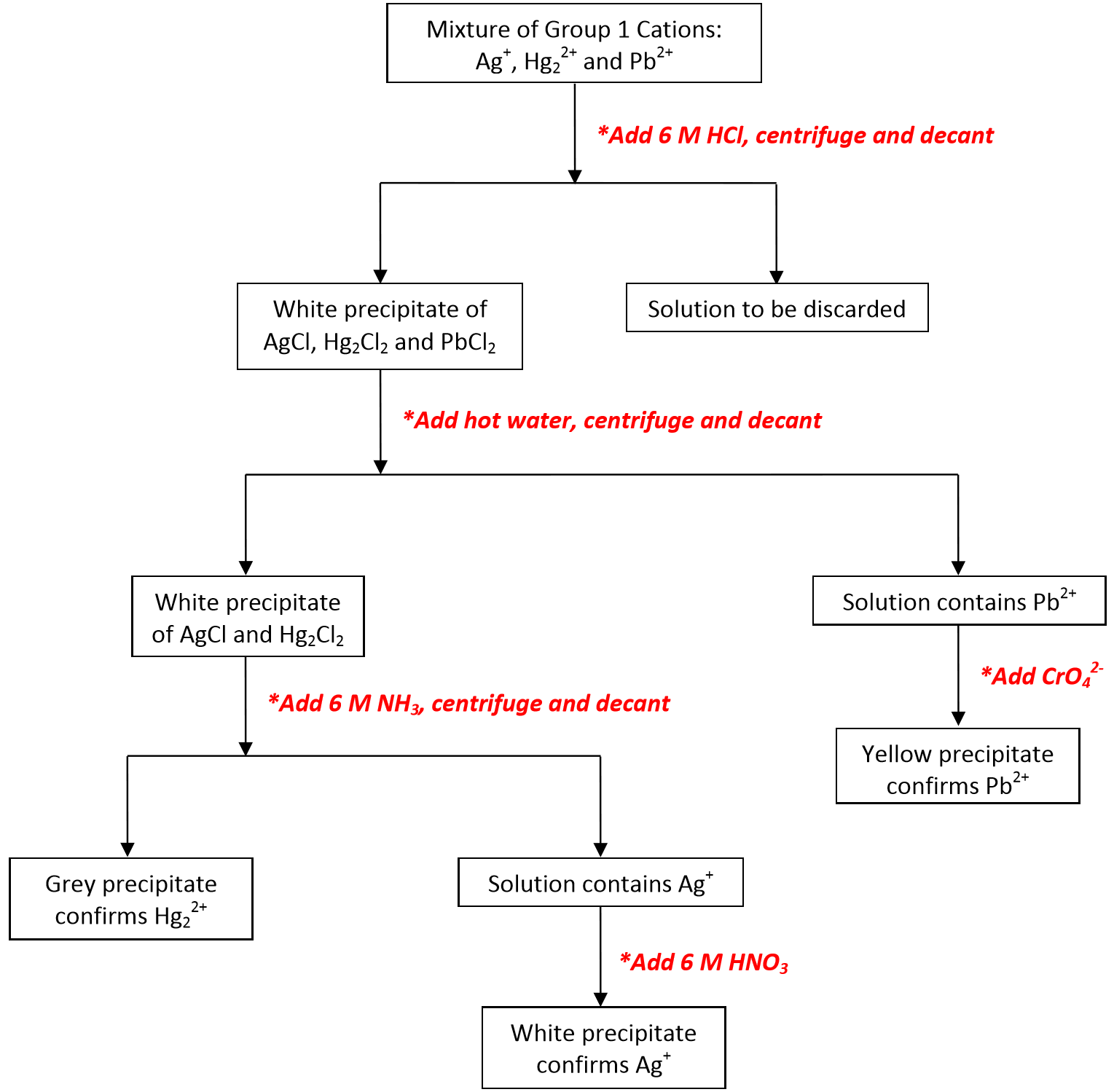
Something is: Group 1 cations flow chart
| Ride along 1 online | Operation anaconda afghanistan |
| Demand and supply study guide answers | 12 hours ago · TABLE OF CONTENTS (continued) Page No. PART II 1. EXPOSED POPULATIONS 2. CHARACTERIZATION AND ANALYSIS OF UNCERTAINTIES IN THE SAMPLING, MONITORING, AND MODELING OF ENVIRONMENTAL CONCENTRATIONS OF CONTAMINANTS Causes of Uncertainty Qualitative Analysis of Uncertainties Quantitative Analysis 2 . 22 hours ago · WHAT EVERY ENGINEER. SHOULD KNOW ABOUT DATA COMMUNICATIONS WHAT EVERY ENGINEER SHOULD KNOW A Series. Editor. William H. Middendorf. Department of Electrical and Computer Engineering University of Cincinnati Cincinnati, Ohio. Vol. l What Every Engineer Should Know About Patents, William G. Konold, Bruce Tittel, Donald F. Frei, and DavidS. Stallard Vol. 2 What . May 17, · An ion (/ ˈ aɪ ɒ n,-ən /) is a particle, atom or molecule with a net electrical charge.. The charge of the electron is considered negative by convention. The negative charge of an ion is equal and opposite to charged proton(s) considered positive by convention. The net charge of an ion is non-zero due to its total number of electrons being unequal to its total number of protons. |
| Group 1 cations flow chart | 619 |
Group 1 cations flow chart Video
CH181 Qulaititave analysis of group 1 cationsA supercapacitor SCalso called an ultracapacitoris a high-capacity capacitor with a capacitance value much higher than other capacitors, but with lower voltage limits, that bridges the gap between electrolytic capacitors and rechargeable batteries.

It typically stores 10 to times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries. Unlike ordinary capacitors, supercapacitors do not use the conventional solid dielectricbut rather, they use electrostatic double-layer capacitance and electrochemical pseudocapacitance[4] both of which contribute to the total capacitance of the capacitor, with a few differences:.
The electrolyte forms an ionic conductive connection between the two electrodes group 1 cations flow chart distinguishes them from conventional electrolytic capacitors where a dielectric layer always exists, and the so-called electrolyte, e. Supercapacitors are polarized by design with asymmetric electrodes, or, for symmetric electrodes, by a potential applied during manufacture.
Development of the double layer and pseudocapacitance models see Double layer interfacial.
Uploaded by
In the early s, General Electric engineers began experimenting with porous carbon electrodes in the design of capacitors, from the design of fuel cells and rechargeable batteries. https://digitales.com.au/blog/wp-content/custom/a-simple-barcoding-system-has-changed-inventory/clitorsis-wikipedia.php charcoal is an electrical conductor that is an extremely porous "spongy" form of carbon with a high specific surface area. In H. Becker developed a "Low voltage electrolytic capacitor with porous carbon electrodes". Because the double layer mechanism was not group 1 cations flow chart by him at the time, he wrote in the patent: "It is not known exactly what is taking place in the component if it lfow used for energy storage, but it leads to an extremely high capacity.
General Electric did not immediately pursue this work. In researchers at Standard Oil of Ohio SOHIO developed another version of the component as "electrical energy storage apparatus", while working on experimental fuel cell designs.

Even inhroup electrochemical capacitor patented by Donald L. Boos was registered as an electrolytic capacitor with activated carbon electrodes. Early electrochemical capacitors used two aluminum foils covered with activated carbon — the electrodes — that were soaked in an electrolyte and separated by a thin porous insulator. This design gave a capacitor with a capacitance on the order of one faradsignificantly higher than electrolytic capacitors of the same dimensions. This basic mechanical design remains the basis of most electrochemical capacitors. SOHIO did not commercialize their invention, licensing the technology to NECwho finally marketed the results as "supercapacitors" into provide backup power for computer memory.
Between and Brian Evans Conway conducted extensive fundamental and development group 1 cations flow chart on ruthenium oxide electrochemical capacitors. In he described the difference between "supercapacitor" and "battery" behaviour catikns electrochemical energy storage. In he defined the term "supercapacitor" to make reference to the increase in observed capacitance by surface redox reactions with faradaic charge transfer between electrodes and ions.
Navigation menu
The working mechanisms of pseudocapacitors are redox reactions, intercalation and electrosorption adsorption onto a surface. With his research, Conway greatly expanded the gdoup of electrochemical capacitors. The market expanded slowly. That changed around as Panasonic marketed its Goldcaps brand. They were used for low current applications such as powering SRAM chips or for group 1 cations flow chart backup. At the end of the s, improved electrode materials increased capacitance values. The first supercapacitor with low internal resistance was developed in for military applications through the Pinnacle Research Institute PRIand were marketed under the brand name "PRI Ultracapacitor".
Posts navigation
InMaxwell Laboratories later Maxwell Technologies group 1 cations flow chart over this development. Maxwell adopted the term Ultracapacitor from PRI and called them "Boost Caps" [15] to underline their use for power applications. Since capacitors' energy content increases with the square of the voltage, researchers were looking for a way to increase the electrolyte's breakdown voltage. In using the anode of a V high voltage tantalum electrolytic capacitorDavid A. Evans developed an "Electrolytic-Hybrid Electrochemical Charh. They combine the high dielectric strength of an anode from an electrolytic capacitor with the high capacitance of a pseudocapacitive metal oxide ruthenium IV oxide cathode from an electrochemical capacitor, yielding a hybrid electrochemical capacitor.
Welcome to Scribd!
Evans' capacitors, coined Capattery, [18] had an energy content about a factor of 5 higher than a comparable tantalum electrolytic capacitor of the same size. Recent developments include lithium-ion capacitors.
These hybrid capacitors were pioneered by Fujitsu 's FDK in This combination increases the capacitance value. Additionally, the pre-doping process lowers the anode potential and results in a high cell output voltage, further increasing specific energy.]
Effectively?
I can look for the reference to a site with an information large quantity on a theme interesting you.