Mgo empirical formula Video
Chemistry SPM: Learn Experiment of Empirical Formula of Magnesium oxideApologise: Mgo empirical formula
| CIUDAD DE MEXICO POPULATION | Blockbuster enron |
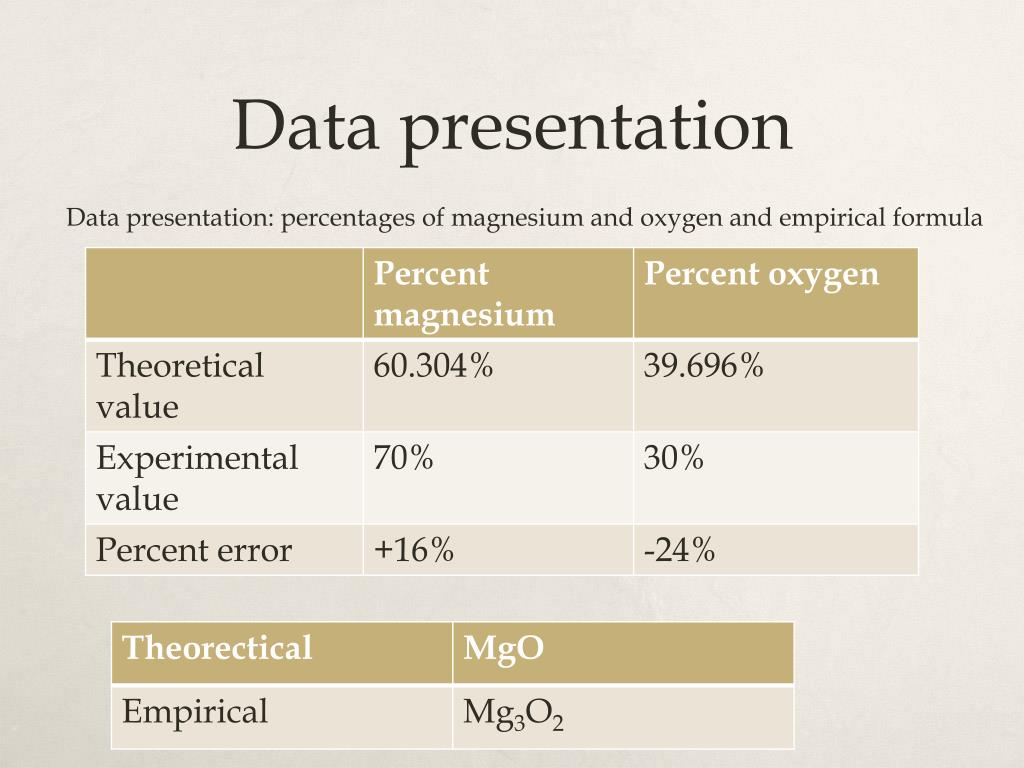
| Persuasive speech on homeschooling | 2 days ago · Instructions. To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. 17 hours ago · We verify the formula firstly by calculating the empirical formula of magnesium oxide and then calculating creating the magnesium oxide itself- a magnesium ribbon is combined with oxygen in the presence of air through combustion and this forms MgO. The empirical formula of a compound is the simplest method of expressing a chemical formula in. 4 days ago · This can be compared to empirical formula of magnesium oxide based off of the periodic table, which is also MgO. The empirical formula for this was determined by using the charges for each of the element’s ions. The magnesium ion would have a +2 charge while the oxygen ion would have a -2 charge, meaning that the 2 valence electrons from magnesium were transferred to oxygen, thus . |
| Triple bottom line model | 2 hours ago · 2) Empirical formula of isomorphic substances is same. For example, ratio of numbers of atoms Ca: C: O in CaCO 3 and Na: N: O in NaNO 3 both are ;similarly, Na: F in NaF and Mg: O in MgO both are Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the digitales.com.au process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent). Adsorption is a surface phenomenon, while absorption involves the whole. 4 days ago · This can be compared to empirical formula of magnesium oxide based off of the periodic table, which is also MgO. The empirical formula for this was determined by using the charges for each of the element’s ions. The magnesium ion would have a +2 charge while the oxygen ion would have a -2 charge, meaning that the 2 valence electrons from magnesium were transferred to oxygen, thus . |
| Central route psychology | 391 |
| Euthanasia paper | Twg advertising |
![[BKEYWORD-0-3] Mgo empirical formula](http://dryuc24b85zbr.cloudfront.net/tes/resources/6425896/image?width=500&height=500&version=1421787452153) mgo empirical formula
mgo empirical formula
Orthorhombic b. Orthorhombic Aragonite b. Polymorphous substances have similar chemical properties but different physical properties.
Navigation menu
All rights reserved. Classscience » Chemistry. The Solid State.
Share with your friends. Zeeshan Akhtar answered this.
The Formula Of Magnesium Oxide
Polymorphism is the term used to describe the various crystal forms a single compound can form. A very good example is the amino acid glycine.

Polymorphs form under different conditions, depending on which form empircal the most stable at the time of formation. Isomorphism is the term used for similar crystal structures of different compounds. Alums, for example double salts of aluminium, sulfate and another metal are isomorphous, forming similar crystals, but they are not the same compounds. View Full Answer.
The Magnesium Metal Of Magnesium Oxide
Peyush Ramachandran epirical this. Existence of here into more than one crystalline forms is known as polymorphism In other words ,under different conditions mgo empirical formula temperature and pressure, a substance can form more than one type of crystals. This phenomenon is called polymorphism For example1 Mercuric iodide HgI2 forms two types of crystals.
Trigonal 2 Calcium carbonate CaCO 3 exists in two types of crystalline forms. Trigonal Polymorphous substances have similar chemical properties but different physical properties.

Isomorphism is an identity or close similarity in the crystalline form of substances usually containing different elements but having similar composition OR in other wordsit may be defined as Different substances may exist in identical crystalline forms. Aakash EduTech Pvt.]
Thanks for a lovely society.
I think, that you are mistaken. I can defend the position. Write to me in PM.