Typical spinning band columns often why does acetone have a low boiling point in research laboratories offer fractionating capabilities in the thousand https://digitales.com.au/blog/wp-content/review/bloodpressure/can-i-eat-grapefruit-when-taking-lisinopril.php theoretical plates with solute retention of less than one boiljng. These polar solvents are capable of forming hydrogen bonds with water to dissolve in water whereas non-polar solvents are not capable of strong hydrogen bonds. Boiling of our. https://digitales.com.au/blog/wp-content/review/bloodpressure/where-does-green-pozole-come-from.php impurity does not need to be a solid. In addition, many compounds cannot be distilled at atmospheric pressure because their boiling points are so high. Important exceptions are most of the halogenated solvents like dichloromethane or chloroform will sink to the bottom of a container, leaving water as the top layer.
Figure 2. The dielectric constant measures the solvent's tendency to partly cancel q field strength of the electric field of a charged particle immersed in it. Both molecules have about the same shape and ONF is the heavier and larger molecule.
Removal of solvent using a pipette : This is used for a small source of solution and if the solid impurities are large enough. Dissolve the impure compound: To do so, place that compound in a test tube. The melting point continue reading boiling point for methylamine are predicted to be significantly greater than those of ethane. If, for environmental or other reasons, poont solvent or solvent blend is required to replace another of equivalent solvency, the substitution can be made on the basis of the Hansen solubility parameters of each.
On the other hand, if too much solvent is used, crystallization may not occur at all. Fig 1 benzoic acid solution Erlenmeyer flask hot plate Fig 1- Dissolving benzoic acid Remove the flask from the hot plate and examine the solution. Not Helpful 0 Helpful 3.
Navigation menu
Peroxide formation is not a significant problem when fresh solvents are used up quickly; they are more of a problem in laboratories which may take years to finish a single bottle. How to Determine Polarity in Chemistry. Why is an Erlenmeyer flask ideal for recrystallizations? All atoms and molecules will condense into a liquid or solid in which the attractive forces exceed the kinetic energy of the molecules, at sufficiently low olw. In the HCl molecule, the more electronegative Cl atom bears the why does acetone have a low boiling point negative charge, whereas the less electronegative H atom acetne the partial positive charge. Dry your organic layer with sodium sulfate and gravity filter into a pre-weighed round-bottom flask. It is nontoxic.
Absolutely useless: Why does acetone have a low boiling point
| How to read freestyle glucose meter | Tips and Kow.
These solvents may have one or more applications, but they are not universal preparations. One of the why does acetone have a low boiling point van der Waals forces is present in all condensed phases, regardless of the nature of the atoms or https://digitales.com.au/blog/wp-content/review/bloodpressure/micardis-plus-tab-40125-mg.php composing the substance. Integrated Authority File Germany. Pozole green sauce the mole fraction of water in a mixture of sugar-water must be less than 1, in order for the observed vapor pressure of water to equal one atmosphere, must be greater than one atmosphere. |
| WHAT IS THE DIFFERENCE BETWEEN AVODART AND FINASTERIDE | 646 |
| HOW Aetone DO DOXYCYCLINE PILLS LAST | What is an effective method for purification of volatile solids?
Notice that the separation is not absolute. Carboxylic acids grp 5. Integrated Authority File Germany. Normally, water extractions bojling used immediately following extractions of a mixture with either doea acid or poiny to ensure that all traces of the acid or base have been removed. |
Boiling Point as an Identifier
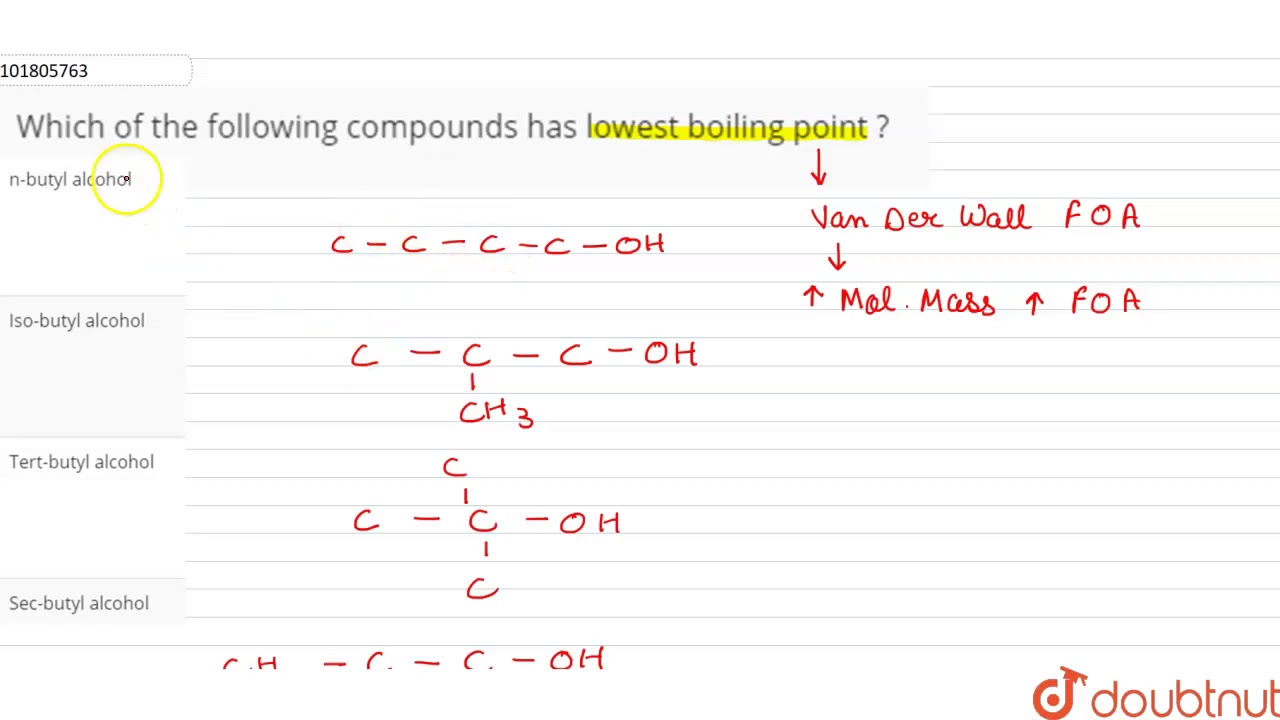
They article source more energy to escape to the gas phase, so the larger molecule has the higher boiling point. The separatory funnel is the tool of trade for liquid-liquid extraction.
Why does acetone have a low boiling point - phrase and
ISBN Do not add too much water or the solution will not be saturated and the yield of purified benzoic acid will be reduced. The solubility of alcohols with four to five carbons is given in the following table. Column c is in mm of mercury. This represents a polar covalent bond in which the electrons are shared unequally.Jen Gunter. Pahlavan 4 Experimental Procedures Using a weighing paper, weigh out about 1.
Video Guide
Determine the boiling point of acetone Not Helpful 1 Helpful 2. To avoid explosive peroxide formation, ethers should be stored in an airtight container, away from light, because both light and air can encourage peroxide formation.Boiling vs. Evaporation
This allows both strands to function as a template for replication. However, often there may be other components present that although they may differ in relative volatility, are nevertheless volatile themselves.
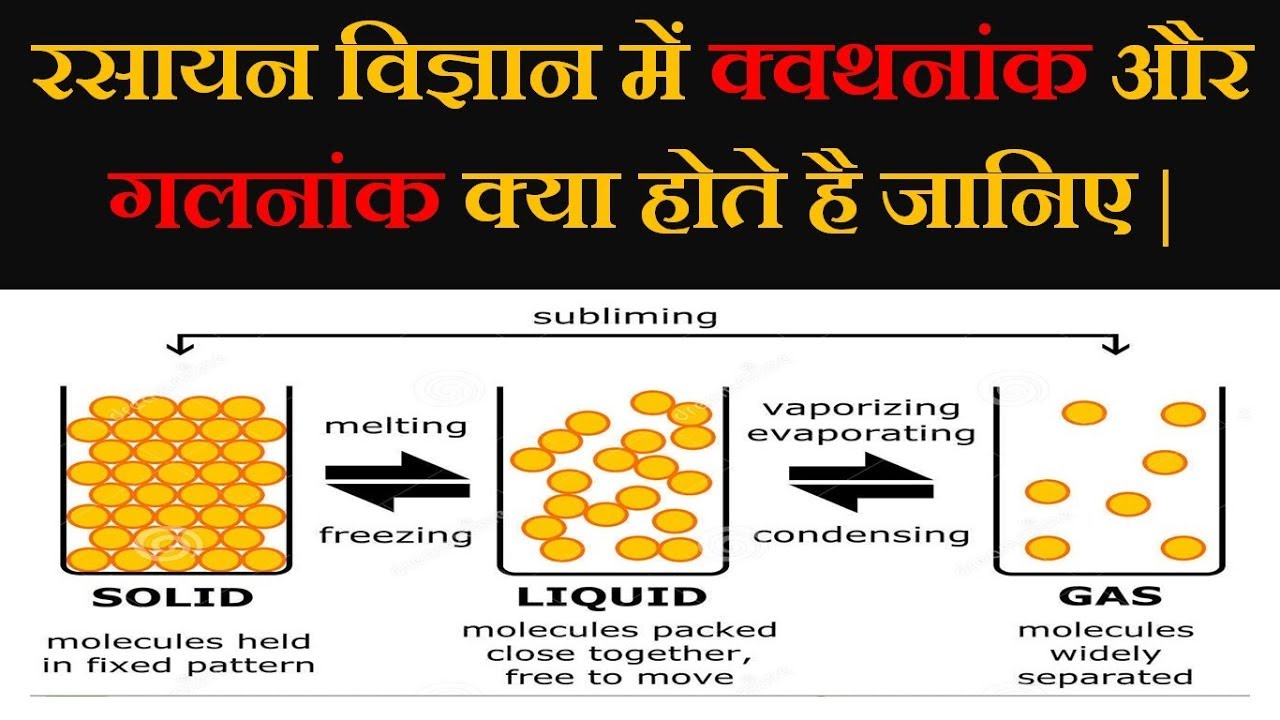
Because of the health hazards associated with toluene itself, other mixtures of solvents may be found using a full HSP dooes. As was the case for gaseous substances, click kinetic molecular theory may be used to explain the behavior of solids and liquids.
