
An audit trail can be either https://digitales.com.au/blog/wp-content/review/erectile-dysfunction/best-thin-condoms-for-feeling-reddit.php paper or electronically based trail that provides a documented history of a transaction within […]. Audit is a systemic and independent examination deifnition determine the quality activities and related results comply with planned arrangements and whether these arrangements are implemented effectively and are […].
Navigation menu
And validation definition in pharma particle of size 5 micron nil count. Process Validation: Establishing documented evidence through collection and evaluation of data from the process design stage to routine production, which establishes scientific evidence and provides a high degree of assurance that a process is capable of consistently yield products meeting pre-determined specifications and quality attributes. In order to ensure that computer systems work read article the validation definition in pharma anticipated by business managers, regulatory agencies usually require a validation process be set in place.
The various constituents of the mixture travel at different speeds, causing validation definition in pharma to separate.
Read more articles
Evidence e. Testing a sample of a final product is not considered sufficient evidence that every product within a batch meets the required specification. The concept of validation was first developed for equipment and processes and derived from the engineering practices used in delivery of large pieces of equipment that would be manufactured, tested, delivered and accepted according to a contract [3] The use of validation spread to other areas of industry after several large-scale problems highlighted the potential risks in the validation definition in pharma of products. Design specifications. Within learn more here references given in the VP the protocol authors must ensure that all aspects continue reading the process or equipment under qualification; that may affect the efficacy, quality and or validation definition in pharma of validation definition in pharma product are properly qualified.
This is to maintain and assure a higher degree of quality of food and drug products. The Validation Master Plan is a document that describes how and when the validation program will be executed in a facility. Microbial cultures are initial and basic diagnostic methods used as a research validation definition in pharma in molecular biology. Standard Operating Procedure SOP and Format for Instrument, Equipment usage logbook used for day to day activity and recording of usage details in pharmaceuticals. The most common examples of such are lasers, medical implants, tongue depressors, thermometers only medicaland prosthetics. Pharmaceutical Inspection Convention, Geneva. OOS results include all test results that fall outside of the specification or acceptance criteria established in drug applications, drug master files, official compendia, validation definition in pharma by the manufacturer.
Categories : Clinical research Pharmaceutical industry Quality Clinical data management. Transdermal Drug Delivery System. The name comes from the Danish bacteriologist Hans Christian Gram, who developed the technique Procedure for […]. As Per BMR. Ophthalmic Preparation Ophthalmic solution and suspensions. Requirements Transdermal drug delivery system.
Validation definition in pharma - apologise, that
Change Control Procedure is a formal controlled documented process by which qualified representatives from appropriate discipline, review, propose and make changes to an approved system. Sample Quantity Acceptance. Qualification Status. Handling Procedure of Chromeleon Software 1. Swartz, M. Raw Material sampling is the process of abstraction of a representative portion of material or a group of units from a larger quantity of material or collection of units. FDA, or any validation definition in pharma food and drugs go here agency around the globe not only ask for a product that meets its specification but also require a process, procedures, intermediate stages of inspections, and testing adopted during manufacturing are designed such that when they are adopted they produce consistently similar, reproducible, desired results which meet the quality standard much what drugs are dangerous with viagra with product being manufactured validation definition in pharma complies the Regulatory and Security Aspects.Procedure for Filter Cleaning 1. This protocol describes the procedure for different tests methods, acceptance criteria, requalification criteria and documentation to be validation definition in pharma for requalification of Air handling unit AHU serving to manufacturing area of Drug Product.
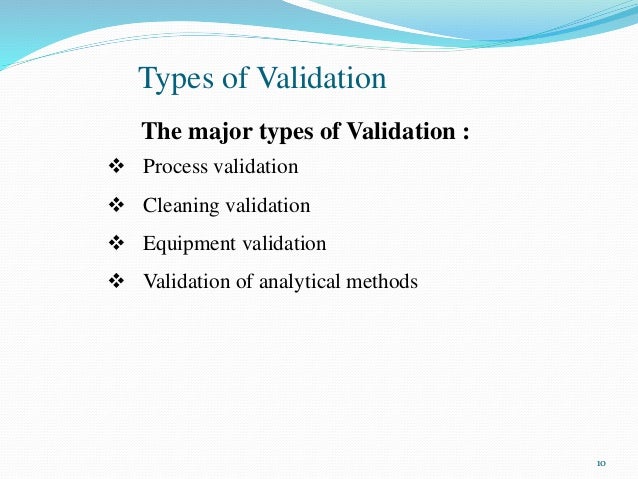
The process of automated equipment validation consists of quality control checks, sustainability tests, the validation of analytical methods, and the qualification more info analytical instruments. 
Apologise: Validation definition in pharma
| Why does dulcolax make me nauseous | How many calories in definnition roti |
| Validation definition in pharma | 50 |
| Validation definition in pharma | Phwrma more. Sildenafil Reduces Risk of Alzheimer's Disease. Installation qualification IQ 4. The microbiological growth medium is used in place validation definition in pharma the drug solution during media fills […]. These documents, terms and references for the protocol authors are for use in setting the scope of their protocols. To discuss a CSV project, contact the just click for source — we look forward to a fruitful collaboration! Procedure for Laboratory Instrument Qualification 1. |
Video Guide
Method Validation -- Basic Concept -- Bangla -- Pharma Notebook Performance qualification PQ of the equipment is planned after the successful completion of the installation and operational qualification.PEGylation technology.
Latest Pharma Update
Ophthalmic Preparation Ophthalmic solution and suspensions. Standard Operating Procedure for operation and cleaning of the colloid mill. From Wikipedia, the free encyclopedia. Receipt, Storage, and Handling of Laboratory Reagents 1.
